In chemical reactions, atoms are never created or destroyed.
The same atoms that were present in the reactants are present in the products as well and are merely reorganized into different arrangements.
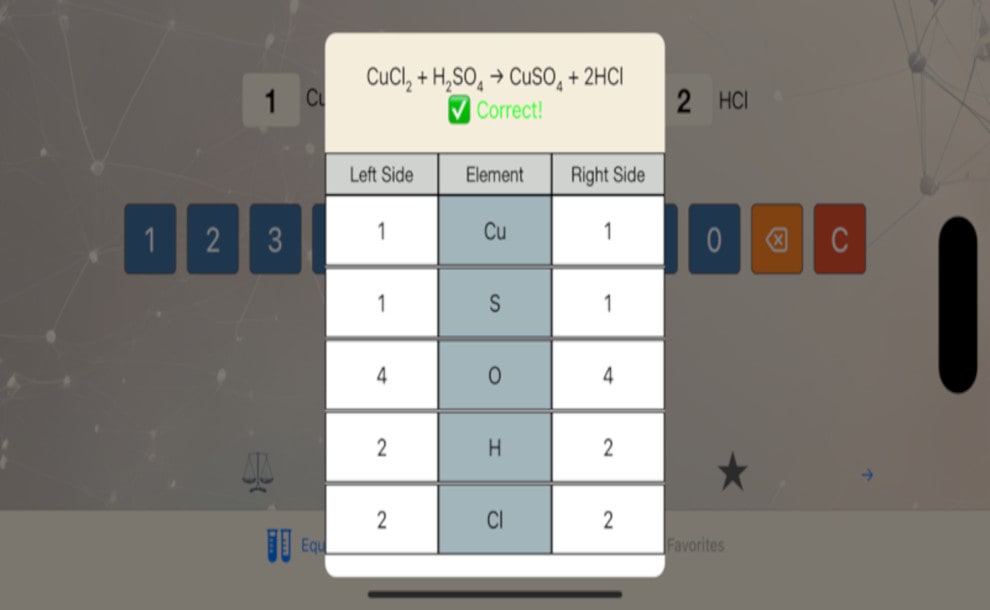
In a complete chemical equation, the two sides (Reactants and the Products) of the equation must be balanced.

Check on App Store
The same atoms that were present in the reactants are present in the products as well and are merely reorganized into different arrangements.
In a complete chemical equation, the two sides (Reactants and the Products) of the equation must be balanced.
Check on App Store
- iphone educational apps